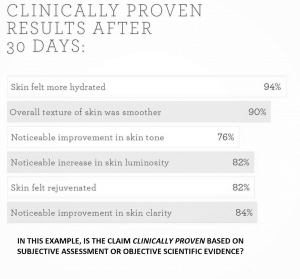
When testing a new cosmetic product or treatment, one sets out to confirm its effects are actually due to the treatment and not to chance or other factors. The most scientifically rigorous way to do this is by comparing it against a placebo or control. Trial design is one of the assessed factors when submitting a paper to any reputable scientific journal – and one must have an extremely compelling reason if they are not using a placebo-controlled design. In order to better design and interpret the results of a placebo-controlled study, this article will provide further insight as to the role of the placebo group (or placebo side, in a split face study) from both researcher and participant perspectives.
Understanding placebo-controlled trials
In a scientific study, the effect of a new product or procedure has to be compared with the effect of another product or procedure, to assess differences in performance. In absence of this comparison, the benefits of the new product or procedure cannot be argued with certainty. It is not enough to, for example, identify a significant change from baseline in a single product. The result could equally be due to random chance, external factors, or well known statistical artefacts such as a regression to the mean.
When a placebo or control is introduced, it establishes a baseline that is subject to exactly the same environmental conditions as the tested product. Any changes over the study in this group will either be due to a combination of random factors, the placebo effect, and the effect of the comparator product if applicable. Therefore, any changes in the treatment group that is excess of the placebo can be confidently ascribed to the product itself having controlled for all other effects.
The only exception where a study should not use a placebo is for those involving life-threatening conditions, and where an already proven drug exists. In these cases, the use of a placebo is considered unethical as it denies patients the usually standard of care they would receive. The use of placebo is ethical and necessary in severe conditions with no efficient drug, in some severe conditions even when an active reference treatment is available, and in all otherwise moderate and functional conditions1.
The impact of the placebo on participants
Studies are generally designed with a specific number of participant and appropriate distribution between active and placebo, in order to conduct statistical analysis of the results. Participants are not obliged to stay in a study and they can willingly decide to withdraw their participation for any reasons. They might stop because of an adverse event, due to a changing schedule, or simply change their mind. Whatever the reason might be, a drop in participant numbers can adversely affect the results of the study.
It is therefore important that if participants decide to take part in a study, they do so after being duly informed of the chance of them being assigned a placebo and why. The Declaration of Helsinki of human rights in medical research, is the current reference guideline about ethics in clinical trials is the, which emphasises three major principles:
- Respect of the patient to accept or not to participate in a trial;
- The constraints and the presumed risks must be acceptable for patients included in a study;
- Vulnerable subjects should not participate in studies1.
Study information provided to participants is quite extensive in explaining what a placebo is and any possible risks associated with the study. Nevertheless, the most common question asked by study participants relates to the logic behind using a product that is possibly a placebo, something that will likely have no appreciable effect. Therefore, perhaps more emphasis could be put on explaining why placebo is needed in the first place and the important role it plays. As most participants can be quite overwhelmed by the amount of information they receive, a simple, easy-to-understand explanation is key here.
Study participants must understand this before entering a study in order to both avoid disappointment and to reduce the incidence of early withdrawals as a result. Both can have a negative effect on overall study recruitment targets. When studies fail to recruit a sufficient number of participants (in both active and placebo groups), the study might have to continue for longer than expected, affecting overall study budget and delaying product launch. Or, the study might fall short and not have enough statistical power to reach a conclusion.
It is indeed common for participants to withdraw from a study due to the lack of benefits perceived in continuing the treatment. From a researcher perspective, we want to praise study participants who diligently stay in a study until the end, where not prevented by otherwise understandable reasons. Regardless of which group they are in, participants should understand their valuable contributions to advancing clinical research.
On the other hand, we would like to see that all studies, big and small, provide more information to participants with regards to the rationale behind the placebo, so they can make an informed decision on whether to stay or not if improvement is not directly perceived. Participants need to understand that without placebo, many great products could not be properly assessed for the benefit they provide compared to what’s already available.
Though it may seem an inconvenience or even a waste of time for study participants, the act of using a placebo is indeed a pivotal element for the study to come to a valid conclusion. Without it, we could indeed be stuck without better treatment options and without hope in overcoming some of the challenges many of us are facing every day. In clinical trials, placebo IS the key to providing better health and personal care, and everyone should know that.
————————–
- Chassany O, Duracinský M. Ethics and clinical trials. Fundam Clin Pharmacol. 1999;13(4):437-44. doi: 10.1111/j.1472-8206.1999.tb00001.x. PMID: 10456284.