The claim clinically proven has become very popular with marketers of both therapeutic and cosmetic products. This is likely due to the fact that this claim may provide consumers with greater confidence in their choice of products.
In fact, although ‘what’ has been clinically proven is not often disclosed, stating that the product is clinically proven suggests that there are proven benefits in using these products, which is enough to induce some consumers to choose these products over others. This is because ordinary consumers might understand a product as having better efficacy, higher safety, or more reliable results.
Breaking down this statement, the word ‘clinical’ refers to the fact that the product has undergone one or more clinical trials, which are studies conducted by a qualified research team in a controlled, scientific manner. The power of the scientific method is that one can be sure any results obtained are not due to external or uncontrolled factors, and if documented properly, such an experiment is repeatable for anyone wishing to independently verify the results.
The word ‘proven’ means that in the clinical trial(s), the results obtained have been demonstrated to be statistically significant. This means that one can be fairly confident that the results of product and a test comparator were not obtained due to random variance (chance), or other extraneous factors.
Despite being used so often these days, the clinically proven claim still generates some confusion among consumers as they do not know exactly what the term entails. Sometimes a contributing factor is the marketers themselves, who may also misunderstand the term and subsequently apply it incorrectly. For complementary medicines, the TGA has some guidance on use of this term, on their website stating that “…These terms are not acceptable unless supported unequivocally by robustly designed, published peer-reviewed clinical trial(s) conducted on the actual medicine being advertised, or an identical formulation and dose (as a minimum)”. For cosmetic products, the lack of guidelines for the use of claims such as clinically proven might have contributed to some common misconceptions as discussed below.
Efficacy rather than safety
I often get asked if the term clinically proven can refer to the product’s safety and whether it can be used when referring to dermatological tests that have proven the product to be suitable for sensitive or delicate skin. Unless the statement clearly refers to product safety (e.g. clinically proven to be safe for all skin types), the answer is no. Used on its own, the term clinically proven refers to the efficacy (i.e. performance) of the product and the benefits it may provide to the skin. It does not refer to the safety of the product or whether the product has the potential to cause a skin reaction (i.e. irritation or sensitization).
Finished product rather than active ingredient
Unless clearly stated, the term clinically proven refers to the finished product (i.e. the complete formulation rather than a specific ingredient). If the finished product has not been tested and shown to be efficacious in clinical trials, this should be made clear by indicating the specific ingredient in the formula that is clinically proven to be effective. Also, to be using the claim clinically proven with regards to the specific ingredient in the formula, the marketer should ensure that the quantity of that ingredient is the same amount used in the clinical trial they refer to. According to the Australian Consumer Protection Law, it would be considered false and misleading to use the statement clinically proven with reference to a finished product when in fact it is based on studies conducted on one of the ingredients only (and the product is made of several other ingredients).
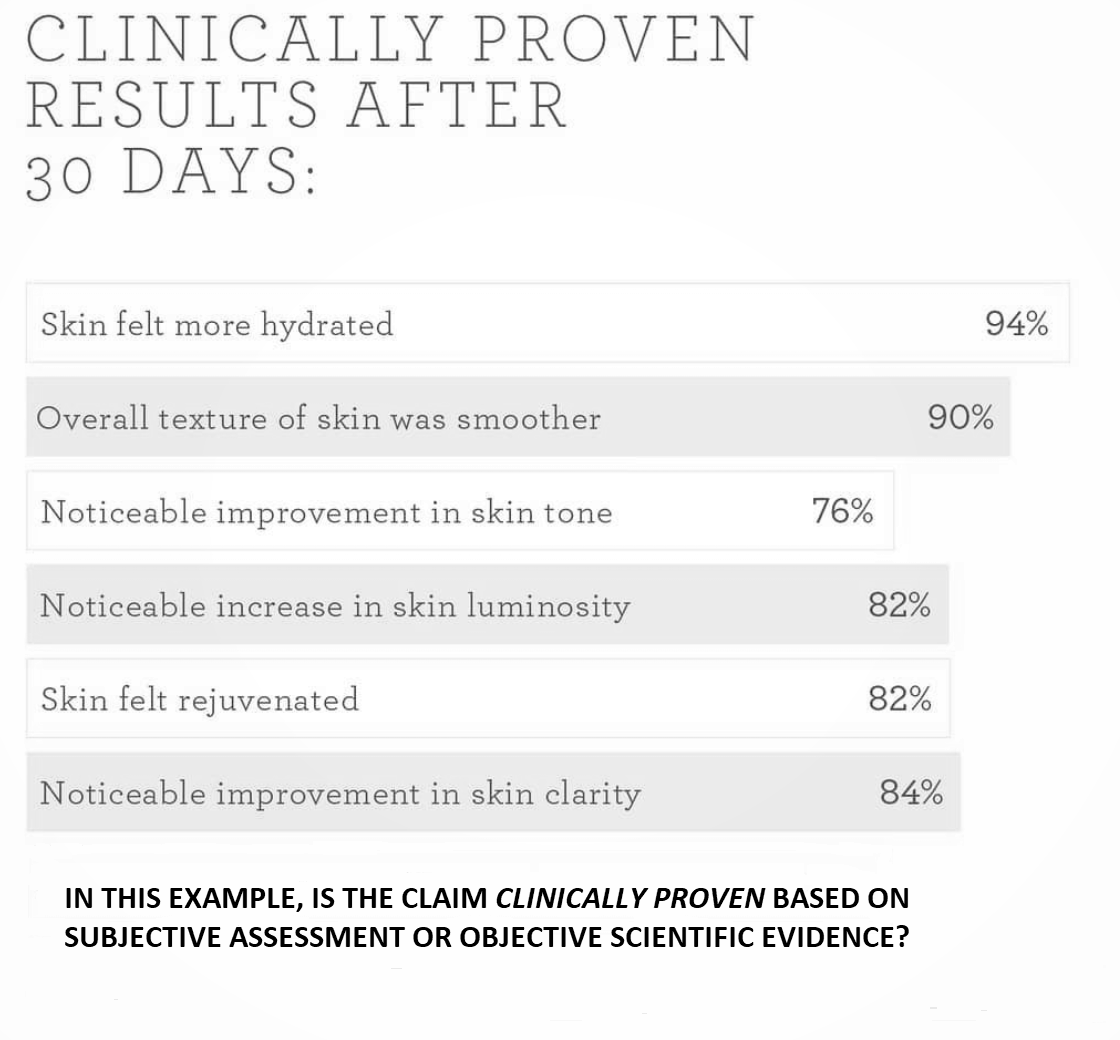
Objective assessments rather than subjective
Another question from cosmetic companies willing to conduct cosmetic trials is whether they can use the term clinically proven with regards to the results from participants questionnaires (subjective assessment). For example ‘X% of subjects though that […] improved’. If the study was solely based on subjective assessment and no objective assessments of any sort were conducted (for example instrumental assessments), then it cannot be said that the results are clinically proven. The reason is that subjective assessment is still fundamentally opinion-driven and while it can be considered valid support to other objective measurements, taken on its own it cannot be considered strong enough evidence to scientifically prove the efficacy of a product.
Educating marketers and consumers
To protect companies that conduct clinical trials and hold proper scientific evidence regarding their products’ efficacy, it is important to educate both consumers and marketers on the work involved to support a clinically proven claim. It is just as important to value the cosmetic companies that invested in scientific studies to determine product efficacy, to acknowledge the time and perseverance of the volunteers that took part in the study, to credit the scrupulosity of the researchers, and the quality of the data collected in clinical studies. In this way, marketers can be truly proud of statements such as clinically proven, and consumers can be confident in that said statements are being used correctly.
References
——————————
https://www.tga.gov.au/book-page/scientific-indications-what-evidence-do-you-need-support-your-scientific-indication