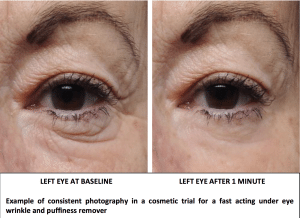
Here is an article featured in the August 2014 issue of The Science of Beauty Magazine about efficacy trials for cosmetic products. This article touches on some basic elements that a scientific cosmetic trial should have in order to have the potential to substantiate efficacy claims.
Did you know that apart from a few exceptions, such as SPF testing, there are currently no standard in vivo efficacy tests for cosmetic products uniform across the industry? Efficacy studies can be purposely designed to suit the specific product, claim and client’s needs.
Although there are some general guidelines for some types of studies, cosmetic testing needs to respond to the specific needs of the sponsor. For this reason, research service providers should have suitably qualified research staff to be able to offer a tailored approach.
Generally the need for an efficacy trial derives from:
- Regulatory requirements – the marketer needs to satisfy local or foreign regulations.
- Marketing – the marketer needs to substantiate a marketing claim in order to comply with marketing and consumer protection laws (ACCC in Australia).
- Research – the marketer needs to establish in vivo efficacy of the product in order to further investigate formulation potential.
Whichever the driving force for the cosmetic trial might be, the key to a successful efficacy study is to define an appropriate study design.
Study design
The gold standard of clinical study design is the randomised controlled study. In a cosmetic study, this means that the treatment area should be assigned at random and compared with at least one control (untreated area, positive control, negative control). Where possible, the study design should incorporate the above key factors as well as method of blinding if relevant (e.g. single blinding, double blinding) to be preferred over open label. Double blind is the most rigorous option, where both the subjects involved in the study and the staff responsible for the assessments are not aware of which area is being treated with the test product.
Study objectives
An essential task is for the study design to clearly define one or more study objectives. The study objective is the research question and the purpose of the entire study (e.g. the objective of the moisturisation test is to determine the moisturising efficacy of the test product applied on the skin of test subjects). At the same time the study design has to be adapted to the specific product. For this reason, it is often correct to say that there can be as many possible study designs as there are different products. Some products are formulated for a specific target market population (age, sex, skin type, skin condition etc.). Some have different mechanisms of action that will help determine whether the length of the study should be short term or long term. All these elements need to be taken into account when designing a study.
Statistical plan
A proper study design has to take into account that a sufficient number of subjects have been enrolled in a study to provide results that are statistically powerful. In other words, if a trial has been conducted on an insufficient number of subjects, the results, although possibly appearing very good, might not be statistically significant to support the overall product’s efficacy. For example it has been observed that the product has had some positive effect on some people but it is not sufficient to indicate that similar results will be experienced by the majority of the people using the product. That is why a sample size calculation based on the primary objective (hypothesis) is normally conducted by the statistical team during the study design phase. Having said that, in some cases, it is possible to base the number of volunteers required for a study on previous trials where similar measurement procedures were used (i.e. taking estimates of expected variance and effect size from historical data). Where sample size calculation is not possible, nor existing historical data is available to determine the sample size, a pilot study should be carried out in the first instance. Once the data for the trial has been collected, the statistical analysis of the results has to take into account the study objective and suitable statistical methods should be employed according to the type of data (i.e. categorical or continuous).
Last, but not least, the study design has to suit the budget allocated by the client.
Standard vs. Customised protocols
Over the years a large number of protocols for testing on cosmetic products have been adopted as “standard” by testing facilities and research providers. This process has often been driven by the desire of the clients to understand, in the first instance, the costs associated with a cosmetic trial. It is particularly true where cosmetic companies have never conducted in vivo efficacy testing before or they are not familiar with trials in general. Such “standard” protocols are better referred to as examples of what a typical scenario might be, and are not necessarily indicative of what the study design and protocol for a particular trial should be. As we have explained earlier, trials on cosmetic and personal care products are experiments (like any other trials), where the research professional is attempting to test an hypothesis (study objective), while keeping in mind the reason for conducting the test, the particular product in question and cost effectiveness for the sponsor. Therefore, although some cosmetic trials can be fairly similar, more often they require some unique elements that the scientific researcher should be able to address during the study design.
Getting it right
There is growing interest in efficacy trials on cosmetic products because of the great impact trials results can have on marketing and product sales. As a result there is a need for well-designed and well-conducted cosmetic trials that can produce proper evidence regarding the efficacy of cosmetic products. Rigorous science based studies are the only ones that set themselves apart in the long run.