Since the increasingly higher use of the internet in the late 90s, people have had access to a variety of resources and information, allowing them to learn about anything and everything. Libraries and training courses, representing a more formal knowledge resource, have lost much of their appeal due to the amount of up-to-date content that can be easily accessed at home. This has created enormous opportunities for many, but also a widespread lack of professional skills and standardisation thereof. Although data can be easily accessed and some concepts might seem deceptively simple, newly acquired knowledge might not be enough without the foundations given by formal education to correctly understand and interpret obtained data.
In the cosmetic industry, this is particularly true during product development. Amateur cosmetic enthusiasts often struggle with getting this part right and will end up bearing the costs. The development of a product cannot be improvised and require technical expertise and sound scientific knowledge at each stage. That is why a professional cosmetic chemist is required. In addition to relying on their expertise, a chemist or cosmetic consultant also understands the safety or efficacy data available for cosmetic ingredients or finished products and will inform their clients if any further data or product testing is required.
Existing data
Cosmetic distributors or manufacturers are able to use pre-existing data on individual ingredients and products with similar formulations. There are several means to access these safety and efficacy data. Raw material suppliers often have data on their ingredients or products. Alternatively, data can be found in cosmetic journals available on platforms such as PubMed and TOXNET, or websites such as the Cosmetic Ingredient Review (CIR). CIR is a reputable industry-funded panel of scientific and medical experts who review the safety of cosmetic ingredients. Naturally, the data will need to be reviewed by a cosmetic professional to understand its validity and relevance to product being developed. If there is not enough data available regarding the safety or efficacy of the ingredients or finished products, then further testing might be necessary.
In vitro
There are a range of lab tests can be conducted to assess products before being tested by humans. Test can be conducted with regards to toxicity and compatibility with human use in general or to establish if they contain any undesired components (allergens, heavy metals, formaldehyde etc.) In vitro testing is also extremely valuable to establish the quality of a finished product and compliance with regulatory requirements (if required). Microbiology testing, stability, and preservative efficacy are just some of the options available to reduce the risk of a recall once the product is on the market. Some in vitro tests are also designed to support product innovation and performance. For example, packaging testing, analysis of actives, or in vitro efficacy testing to support early-stage development (prior to in vivo studies confirming the results).
In vivo
Once a product is deemed to be safe for human use based on the data already available or additional testing undertaken, in vivo studies may be performed. An in vivo study’s primary aim is to confirm or further evaluate the effect and tolerability of the cosmetic product when they are applied on real human skin. In vivo studies can include instrumental testing, user perception studies, and clinical trials. With a few exceptions (e.g. patch testing or dosage testing), in vivo product testing tents to resemble real life scenarios. That is, the product is applied to the area of the skin it is marketed for and in the amount to be recommended to the final consumer.
Clinical trials
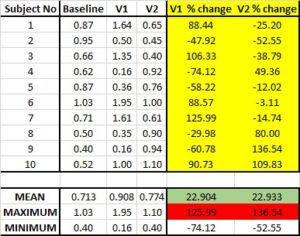
The last step of the data gathering process. A clinical trial is essentially an in vivo study designed to assess the specific effect and safety of products in a more rigorous manner. Not all cometic products will require clinical trials, unless there is the need to substantiate a particular effect (or safety aspect) that cannot be determined with the data already available. As our company specialises in in vivo studies, we sometime receive enquiries from companies that are interested in conducting clinical trial before having any data on their products from existing resources or in vitro testing. This risky and expensive practice might be avoided by engaging a professional in the product development stage.
Safety and efficacy data is essential for the success of a consumer product. As many of us are aware, some of the most popular cosmetic products on the market and their longevity is backed by extensive safety and efficacy data. These are the primary goals of the product developers when developing a new product. Using free data resources responsibly and relying on professionals for further insight is the best combination that will maximise your chances for a successful product launch.
——————————–
References
https://www.fda.gov/cosmetics