When making claims about a cosmetic product, it is the responsibility of the marketer ensure that rigorous scientific evidence supports the claims being made1. The marketer must have sufficient information for each claim being made. The data must be clear, accurate, and relevant to the product, ingredient, or combination of ingredients being marketed. Evidence to substantiate claims can be obtained in different ways. It can be a reference to well-established knowledge or previous research, or be the result of a new study designed for such a purpose. The type of claim to be validated will influence the study design on a case-by-case basis. As such, the quality of the study design, data, and results are crucial.
Below, we will touch on three examples involving evidence in support of cosmetic claims where some improvements need to be made.
QUALITY OF THE EVIDENCE
Clinical trial reports are where the evidence accumulated over a trial is presented in order to support cosmetic claims. It is expected that reports are prepared and checked by clinical research professionals with the appropriate qualifications, experience and skills. While mistakes can happen, these are generally picked up quickly and promptly corrected. However, a common error that has occurred in some of the cosmetic trials reports we have reviewed over the years is the way that average change scores are calculated from the raw data.
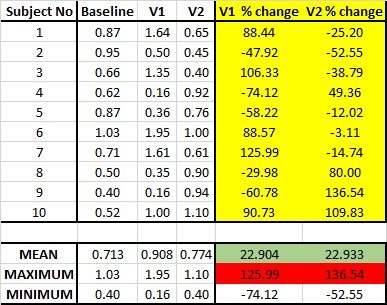
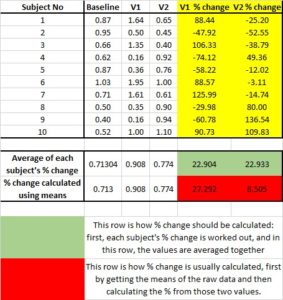
The erroneous way to calculate the average change is to average raw values across visits, and then subtract one mean from the other. Instead, what must be done is to calculate the change on a subject-by-subject basis FIRST, and then get the mean of the individual change scores. This is demonstrated in the table below. The two rows at the bottom compare the ways that change is calculated. As you can see, the two methods of calculation can yield very different results. Remember: you are calculating the average change and NOT the change between averages.
INTERPRETATION AND COMMUNICATION OF DATA
One source of debate in the cosmetic industry has been how to use science to support product claims. Discussions between both R&D and marketing teams are usually the basis for developing a cosmetic claim. The biggest challenge is how one can present information that maintains scientific integrity in a way that consumers can easily understand and relate to.
You might have noticed cosmetic claims referring to a product effect of “up to” X%, which, from the point of view of clinical research professionals seems a rather questionable approach. Commonly, when referring to a product’s efficacy, researchers refer to the ‘average’ effect, which is the average effect one would expect to experience based on the results from the study participants.
On the other hand, it seems that cosmetic marketers making cosmetic claims tend to put more emphasis on the ‘maximum’ effect experienced by a single study participant. Naturally, this is because the ‘maximum’ effect of one person looks better than the ‘average’ effect involving all study participants. For a similar reason, the ‘minimum’ (perhaps even ‘negative’) effect which might be experienced by other test participants (perhaps even the majority of them), is most likely omitted.
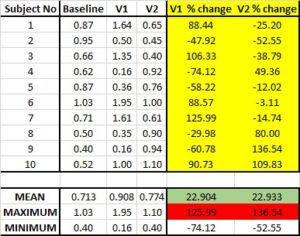
One can argue that referring to product efficacy ‘up to’ is a misrepresentation of the actual product benefits and that a more genuine interpretation of the results should be encouraged. It is used because it gives the impression of better product efficacy, but instead should be treated as the maximum potential of a product. Much like the lottery which advertises the maximum prize pool, it says nothing of what you are most likely to receive if you participate which ignores the majority of the data collected in the study. See the example below where the average effect is highlighted in green while maximum effect is highlighted in red.
CLAIMS ABOUT INDIVIDUAL INGREDIENTS
Many companies developing and selling raw material invest a lot of time and resources into product research, including consumer studies and clinical trials. Most ingredients already on the market will have a product information sheet including all available data on the products’ sensory properties, efficacy and safety. New ingredients naturally require new studies to investigate any benefits they might confer to the user.
The data collected on a specific ingredient enables raw material suppliers to sell their product and can also be used by cosmetics distributors to make claims based on one or more elements included in the product’s formula. An example of an ingredient claim is “contains glycerine to improve skin hydration”. However, a common mistake in cosmetics marketing is to attribute the efficacy of a single ingredient to the finished product. A finished cosmetic product is usually made of several ingredients which may not have ever been tested together in that particular formulation.
Data related to a certain raw material are likely to be quite different from the data related to several ingredients – when used in combination, the properties of various compounds (active or not) are very rarely additive. Therefore, the effects of a single ingredient are not necessarily going to be a representation of the properties of a product containing it along with several other raw materials. It important that this is made clear to the consumer, as portraying the effect of an ingredient as the effect of the finished product is incorrect. Ideally, clinical tests should be run on the product as a whole.
CONCLUSIONS
Responsible product developers and marketers of cosmetic products should place special consideration in the collection, analysis, and presentation of the evidence needed to support their claims. Both industry professionals and regulators need to identify the valid and significant evidence from which claims are derived. Furthermore, these claims must clearly and accurately convey this information to consumers. Having robust scientific evidence in support of cosmetic claims, and the ability to present them correctly will help protect the brand integrity, properly espouse the benefits of cosmetic products, and avoid costly remedies or lengthy legal actions when evidence is flawed, misconstrued or simply not available.
References:
——————————————-
- Joachim W. Fluhr, Practical Aspects of Cosmetic Testing, 1st Edition. 2010.