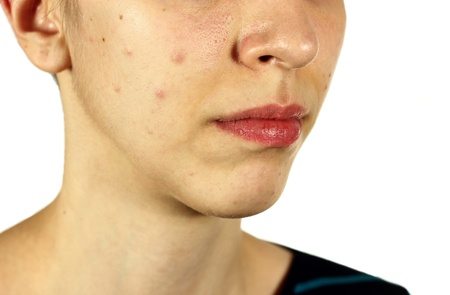
Comedogenicity in cosmetic products has been analysed for a long time, but it has been more extensively debated especially in recent years. Consumers and skin care professionals have been paying more attention to ingredient labels and have been looking for terms such as “non comedogenic” or “non clogging,” hoping that these products will not affect their acne-prone skin. But what does the term “non comedogenic” mean? Does it hold any scientific validity? A closer investigation reveals that comedogenicity has been quite a controversial issue.
“ACNE COSMETICA”
Consumers are sometimes concerned about the effects of cosmetic products on their skin, particularly their potential to increase the incidence of acne. The term “acne cosmetica” was introduced in 1972 by dermatologists Albert M. Kligman and Otto H. Mills to represent the post-adolescent acne characterised by closed comedones, which they believed was caused by cosmetic use. Kligman and Mills were the first to introduce to the cosmetic industry the requirement to test cosmetic ingredients for their potential to increase the incidence of comedones. In response to this new need, an animal test named “the rabbit ear comedogenicity model” was developed, which involved applying the raw material and a control to each rabbit ear for 5 consecutive days for 2 weeks. Visual observation and biopsies followed to assess comedogenicity of the test materials. The “rabbit ear” test was based on a measurable 0-4 point scale of comedogenicity (0= none; 1= trace; 2= mild; 3= moderate; 4= severe).
ANIMAL TESTING MODEL
However, several issues were identified with the “rabbit ear” model. In particular, the fact that the rabbit ear may not accurately simulate the human face. Also, using the animal model on raw materials was based on the assumption that finished formulations containing these ingredients would also be comedogenic. Following this principle, cosmetic industry professionals and consumers were presented with lists of substances to avoid because of the risk to develop comedones. On the contrary, Kligman himself later clarified that “substances that are strongly comedogenic when tested neat (i.e. raw) or in high concentrations become non-comedogenic after sufficient dilution”. Therefore testing the finished product rather than the raw ingredient is much more predictive of the true findings.
HUMAN TESTING MODEL
Due to the concerns raised in regards to the validity of the animal model, and more recently the ban on animal testing for cosmetic products in several countries, scientists have developed a human model for testing finished products. The objective of the human test was to observe the products’ potential to cause comedones by applying a standard amount of test products on a small area (i.e. patches) on the back of subjects with skin prone to comedone formation. Sebaceous follicles are then assessed by a non invasive “follicular biopsy” technique, employing a fast-setting cyanoacrylate glue to remove the follicular contents. Through the cyanoacrylate biopsies it is possible to determine the number of follicles and microcomedones per square centimetre. This model allows testing of the comedogenicity of multiple products compared to a non-treated area. In the 4 point scale used, each test product is compared with the non-treated control for the same person (0= non comedogenic; 1=small microcomedones; 2= moderately sized microcomedones; 3= large microcomedones). The grades of the entire group of study participants are then averaged to give a mean rate of comedogenicity for each product. For this human test it’s important to enrol subjects that are prone to acne and break-outs as comedogenicity is mostly related to this type of skin. Threrefore, the presence of microcomedones at the screening visit is considered one of the inclusion criteria.
TEST “IN USE”
In recent years further considerations have been made regarding comedogenicity testing in humans. A new “in use” cosmetic testing method has been suggested that may overcome some of the inadequacies of the other testing models. “In use” cosmetic tests are based on ‘real life’ scenarios where the test site is the one intended for the product. One of the advantages of testing the product on the face is that it allows the investigator to gather safety information including clinical signs of irritation, symptoms of burning, stinging, itching or tightness occurring during the test, using the skin area for which it was designed. This type of test also allows use of the product as recommended (e.g. application once or twice a day) as well as evaluations regarding combined use of certain products as a regimen. The human “in use” facial test is therefore preferred in terms of clinical significance of the results.
RELEVANCE WITH RESPECT TO ‘FINISHED’ PRODUCTS
Comedogenicity is a concept that was introduced in dermatology and the cosmetic industry several decades ago. It recently gained more interest and it is still prevalent in marketing materials for cosmetic products. Since final formulations are made of several ingredients the initial concept of grading the raw materials for their comedogenicity potential is no longer considered valid. What really matters with regards to comedogenicity is the finished product and how it is used (e.g. how often, alone or in combination).
The review of various testing models adopted over the years, including the animal assay, the human assay on participants’ back and more recently the facial “in use” study, reveals that the latter is generally to be preferred as it offers the most accurate and useful information in predicting comedogenicity and acnegenicity potential.
References
—————————————————————————————–
- Comedogenicity—A Complicated Conversation. Michelle Calvarese, PhD , November 2014 issue of Skin Inc. magazine
- Petrolalum is not comedogenic in rabbits or humans: A critical reappraisal of the rabbit ear assay and the concept of “acne cosmetica” . Albert M. Kligman, Department of Dermatology, University of Pennsylvania School of Medicine, Philadelphia, PA 19104.
- Comedog enicity testing of cosmetics. Zatulove A, Konnerth NA, Cutis. 1987 Jun;39(6):521.
- Comedogenicity in rabbit: some cosmetic ingredients/vehicles. Nguyen SH1, Dang TP, Maibach HI, Cutan Ocul Toxicol. 2007;26(4):287-92.
- A re-evaluation of the comedogenicity concept. Draelos ZD1, DiNardo JC, J Am Acad Dermatol. 2006 Mar;54(3):507-12.
- Acne Cosmetica. Zoe Diana Draelos, Pathogenesis and Treatment of Acne and Rosacea, pp.265-270.
- A Human Model for Assessing Comedogenic Substances. Otto H. Mills, Archives of Dermatology 118(11):903 · November 1982.
- Assessing Comedongenicity: Current and Future Trends. Otto H. Mills Jr. and Richard S. Berger, Clinical Safety and Efficacy Testing of Cosmetics 1990.